and I wasn't going to post this... but since I figure that pretty much no-body reads this I may as well...
I've started to make a guitar from my projects list...
so expect photos, cad draws and such in the sometime near future...
speaking of cad drawings....
www.freecad.com have some free cad programs for those that are interested...
I particularly like Turbocad 4 LE (learning edition), it works pretty much exactly the same as the turbocad that i learned in school.
I tried Autocad 2007 the other day, I find that far too difficult...
strangely though, I do quite like using Pro Engineer for 3d work as I learned how to use that whilst in Uni.
(but neither pro engineer or autocad are free. turbo cadLE (and a load of others I havn't tried), are free.
Thursday, November 30, 2006
The Things I do for a laugh...
It's been a while since I posted anything, and as anyone who actually read this may have guessed...
that's because I havn't made anything...
well that's not strictly true, I havn't made anything worth writing about...
I have started a few projects though.
for instance...
I'm making a tab welder, it's very simple, heres the theory.
welding is a simple process, you take two metals, heat them till they melt, when they have melted the mix, as they cool they harden, attached to each other...
a tab welding welds thins strips of metal together by momentarily melting them with some very high current, they are normally used to join plates to batteries when making a battery pack.
the long and the short of it is that you charge a capacitor, then you discharge it through the surfaces that you want to weld, the capacitor discharges very quickly, and because the resistance of the materials is so low, a large amount of current is draw from the capacitor, large current = large heat, large heat = metal melted and fused...
simple huh?
Anyone who is still confused should google "spot welding",
well... when I say capacitor, I actually mean capacitor bank. I'm using 33,000 uF caps, and plan to have about 200 in my cap bank, simply because I have a regular supply of junk equipment that use these and I can take them out prior to disposal...
it's all about keeping the costs down...
at the moment, (with only 30 caps), I can attach a single strand of copper wire to a slot blanking plate from a computer case...
so I'm hoping that a couple of hundred caps will enable me to weld things from the reasonably thick, to the very thin... altering the amount (time) of current available with the amount I charge the cap bank to.
that's because I havn't made anything...
well that's not strictly true, I havn't made anything worth writing about...
I have started a few projects though.
for instance...
I'm making a tab welder, it's very simple, heres the theory.
welding is a simple process, you take two metals, heat them till they melt, when they have melted the mix, as they cool they harden, attached to each other...
a tab welding welds thins strips of metal together by momentarily melting them with some very high current, they are normally used to join plates to batteries when making a battery pack.
the long and the short of it is that you charge a capacitor, then you discharge it through the surfaces that you want to weld, the capacitor discharges very quickly, and because the resistance of the materials is so low, a large amount of current is draw from the capacitor, large current = large heat, large heat = metal melted and fused...
simple huh?
Anyone who is still confused should google "spot welding",
well... when I say capacitor, I actually mean capacitor bank. I'm using 33,000 uF caps, and plan to have about 200 in my cap bank, simply because I have a regular supply of junk equipment that use these and I can take them out prior to disposal...
it's all about keeping the costs down...
at the moment, (with only 30 caps), I can attach a single strand of copper wire to a slot blanking plate from a computer case...
so I'm hoping that a couple of hundred caps will enable me to weld things from the reasonably thick, to the very thin... altering the amount (time) of current available with the amount I charge the cap bank to.
Tuesday, September 26, 2006
Water Electrolysis
Water Electrolysis
OK...
So I was fairly bored over some time on the weekend, and i decided that i'd have a go at something from my projects list...
something easy, a nice one to start with...
water electrolysis...
basically, what happens, is that you start off with water, an ionic bonded molecule of hydrogen and oxygen... (H2O)
Then you split it into it's base elements, (hydrogen and oxygen) by means of electricity...
anyone who has done simple GCSE science, should have seen this done in the lab, (most likely with the teachers supervision).
Basically what you need for the very simple version, is...
2 electrodes
1 coke bottle
some tape
some wire
a power source, (a 6v battery is a good one)
and some matches, -not strictly needed, but what's the point of generating gasses if you're not going to do burn tests!
This is very simple, (in fact so simple that I'm not including pictures).
Firstly, fill the bottle with water -fill it just above below the very cap of the bottle so there is very little space between the top of the water and the brim of the bottle.
Pure distilled water does not conduct, so if this is an industrial application then things are added, such as Sulphuric acid, or salt... in practice tap water is not pure distilled water, and it works fine. perhaps with less efficiency, but it does work...
anyway, fill the bottle, with water, (or a water-acid/water-salt mix).
Now put the two electrodes in, (long bits of wire, (like a cut up coat hanger) work really well... (in industry they tend to be platinum due to it's hugely inert properties)
Make sure they aren’t touching, and tape then in place to the top...
Now clip some wire onto them and connect the wire to a power source (6v battery).
You should now see bubbles starting to rise to the surface,
(one electrode will bubble seemingly more than the other, that's the hydrogen gas being produced, you'll also notice that it rises faster and with smaller bubbles)...
The other electrode bubbles slightly.
The gas rises to the surface and is just let off...
Light a match and hold it to the top of the bottle, just above the surface of the water. The bubbles that are rising to the top should pop on the surface releasing pockets of hydrogen gas... these pockets should exploded on contact with the naked flame with a small popping sound... (Squeaky pop test for hydrogen).
You can try to collect these gasses in a balloon, to get more volume of gas collected.
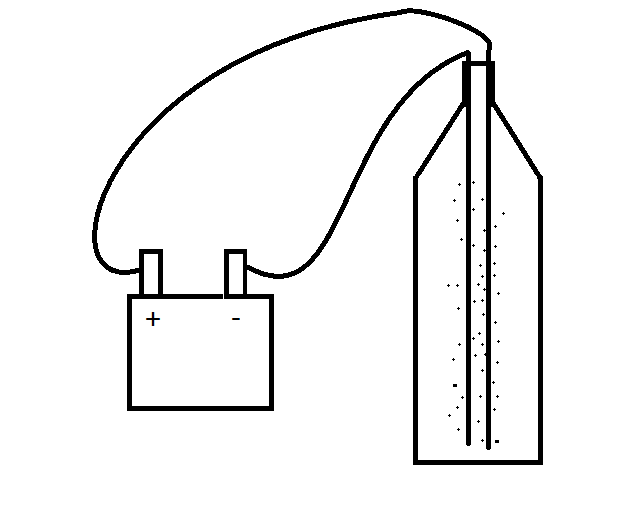
Congratulations,
You're setting fire to a mix of hydrogen and oxygen in your own kitchen...
Warnings...
Oxygen is very very flammable.
Hydrogen can be explosive. (one word... Hindenburg -thought that's not really conclusive either!!)
together Hydrogen and Oxygen together are used as rocket fuel, -you've been warned... -popping the small steady stream of bubbles coming off the top seems perfectly safe...
Collecting the gasses in a large space full of the gasses and then setting light to them will probably not be a great thing to do.
(probably more to do with the oxygen than the hydrogen).
Other warnings.
You can put more power than 6 volts into the contraption. (and it does bubble faster).
I put 24 volts in using the -12 and 12 volt power rails from a PC power supply,
that power supply is now dead, I didn't bother to measure the resistivity to find out what sort of current would be drawn... now I need to make a (another) bench supply...
What you will have noticed is.
Hydrogen burns, Oxygen burns, but from tap water, with a simple 5, 6 or 12volts source it's not generated in significant quantities.
and that's exactly why water electrolysis machines that bolt onto cars just don't work. you don't get nearly enough gas to actually run an engine.
splitting the liquid into it's component forms takes more energy that you get from burning the gasses! so it's never going to power your car, or save you fuel.
A little more complicated set-up can separate the gasses, what you need is two tubes, that are connectted at the bottom (below the electrodes) so that electricity can flow through the water, but the rising gasses won't go through the connection.
At this point Hydrogen will bubble up one tube (ready for you to collect in the top), Oxygen will bubble up the other tube. taps on the top of the tubes can seal them such that the gasses can't escape until you're ready to release the gasses.
A long tube connected to the bridge at the bottom can be used to top up the water level as you generate the gasses.
doing this in either glass or plastic will require special attention to make it all leak proof and gass tight of course.
Here's a rough picture of what your set-up will end up like.
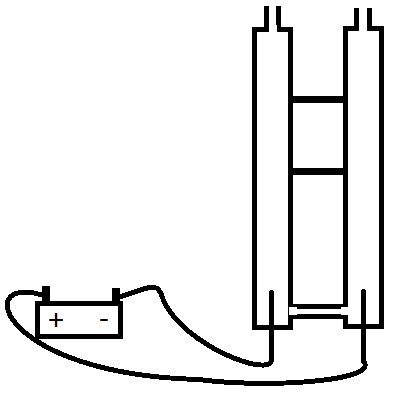
OK...
So I was fairly bored over some time on the weekend, and i decided that i'd have a go at something from my projects list...
something easy, a nice one to start with...
water electrolysis...
basically, what happens, is that you start off with water, an ionic bonded molecule of hydrogen and oxygen... (H2O)
Then you split it into it's base elements, (hydrogen and oxygen) by means of electricity...
anyone who has done simple GCSE science, should have seen this done in the lab, (most likely with the teachers supervision).
Basically what you need for the very simple version, is...
2 electrodes
1 coke bottle
some tape
some wire
a power source, (a 6v battery is a good one)
and some matches, -not strictly needed, but what's the point of generating gasses if you're not going to do burn tests!
This is very simple, (in fact so simple that I'm not including pictures).
Firstly, fill the bottle with water -fill it just above below the very cap of the bottle so there is very little space between the top of the water and the brim of the bottle.
Pure distilled water does not conduct, so if this is an industrial application then things are added, such as Sulphuric acid, or salt... in practice tap water is not pure distilled water, and it works fine. perhaps with less efficiency, but it does work...
anyway, fill the bottle, with water, (or a water-acid/water-salt mix).
Now put the two electrodes in, (long bits of wire, (like a cut up coat hanger) work really well... (in industry they tend to be platinum due to it's hugely inert properties)
Make sure they aren’t touching, and tape then in place to the top...
Now clip some wire onto them and connect the wire to a power source (6v battery).
You should now see bubbles starting to rise to the surface,
(one electrode will bubble seemingly more than the other, that's the hydrogen gas being produced, you'll also notice that it rises faster and with smaller bubbles)...
The other electrode bubbles slightly.
The gas rises to the surface and is just let off...
Light a match and hold it to the top of the bottle, just above the surface of the water. The bubbles that are rising to the top should pop on the surface releasing pockets of hydrogen gas... these pockets should exploded on contact with the naked flame with a small popping sound... (Squeaky pop test for hydrogen).
You can try to collect these gasses in a balloon, to get more volume of gas collected.

Congratulations,
You're setting fire to a mix of hydrogen and oxygen in your own kitchen...
Warnings...
Oxygen is very very flammable.
Hydrogen can be explosive. (one word... Hindenburg -thought that's not really conclusive either!!)
together Hydrogen and Oxygen together are used as rocket fuel, -you've been warned... -popping the small steady stream of bubbles coming off the top seems perfectly safe...
Collecting the gasses in a large space full of the gasses and then setting light to them will probably not be a great thing to do.
(probably more to do with the oxygen than the hydrogen).
Other warnings.
You can put more power than 6 volts into the contraption. (and it does bubble faster).
I put 24 volts in using the -12 and 12 volt power rails from a PC power supply,
that power supply is now dead, I didn't bother to measure the resistivity to find out what sort of current would be drawn... now I need to make a (another) bench supply...
What you will have noticed is.
Hydrogen burns, Oxygen burns, but from tap water, with a simple 5, 6 or 12volts source it's not generated in significant quantities.
and that's exactly why water electrolysis machines that bolt onto cars just don't work. you don't get nearly enough gas to actually run an engine.
splitting the liquid into it's component forms takes more energy that you get from burning the gasses! so it's never going to power your car, or save you fuel.
A little more complicated set-up can separate the gasses, what you need is two tubes, that are connectted at the bottom (below the electrodes) so that electricity can flow through the water, but the rising gasses won't go through the connection.
At this point Hydrogen will bubble up one tube (ready for you to collect in the top), Oxygen will bubble up the other tube. taps on the top of the tubes can seal them such that the gasses can't escape until you're ready to release the gasses.
A long tube connected to the bridge at the bottom can be used to top up the water level as you generate the gasses.
doing this in either glass or plastic will require special attention to make it all leak proof and gass tight of course.
Here's a rough picture of what your set-up will end up like.

Subscribe to:
Posts (Atom)