OK...
So I was fairly bored over some time on the weekend, and i decided that i'd have a go at something from my projects list...
something easy, a nice one to start with...
water electrolysis...
basically, what happens, is that you start off with water, an ionic bonded molecule of hydrogen and oxygen... (H2O)
Then you split it into it's base elements, (hydrogen and oxygen) by means of electricity...
anyone who has done simple GCSE science, should have seen this done in the lab, (most likely with the teachers supervision).
Basically what you need for the very simple version, is...
2 electrodes
1 coke bottle
some tape
some wire
a power source, (a 6v battery is a good one)
and some matches, -not strictly needed, but what's the point of generating gasses if you're not going to do burn tests!
This is very simple, (in fact so simple that I'm not including pictures).
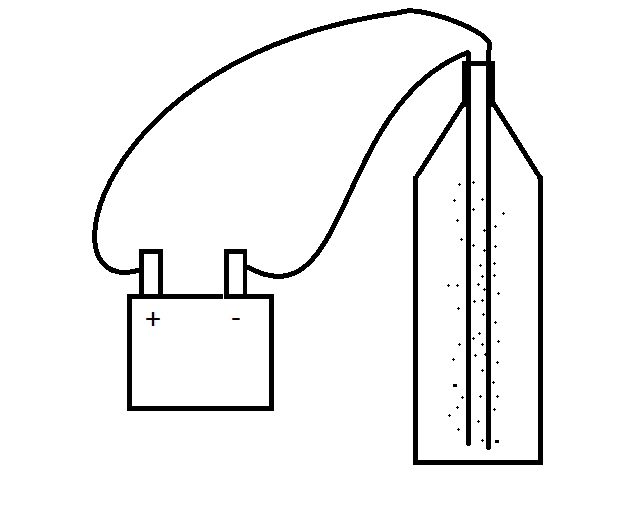
Firstly, fill the bottle with water -fill it just above below the very cap of the bottle so there is very little space between the top of the water and the brim of the bottle.
Pure distilled water does not conduct, so if this is an industrial application then things are added, such as Sulphuric acid, or salt... in practice tap water is not pure distilled water, and it works fine. perhaps with less efficiency, but it does work...
anyway, fill the bottle, with water, (or a water-acid/water-salt mix).
Now put the two electrodes in, (long bits of wire, (like a cut up coat hanger) work really well... (in industry they tend to be platinum due to it's hugely inert properties)
Make sure they aren’t touching, and tape then in place to the top...
Now clip some wire onto them and connect the wire to a power source (6v battery).
You should now see bubbles starting to rise to the surface,
(one electrode will bubble seemingly more than the other, that's the hydrogen gas being produced, you'll also notice that it rises faster and with smaller bubbles)...
The other electrode bubbles slightly.
The gas rises to the surface and is just let off...
Light a match and hold it to the top of the bottle, just above the surface of the water. The bubbles that are rising to the top should pop on the surface releasing pockets of hydrogen gas... these pockets should exploded on contact with the naked flame with a small popping sound... (Squeaky pop test for hydrogen).
You can try to collect these gasses in a balloon, to get more volume of gas collected.
Congratulations,
You're setting fire to a mix of hydrogen and oxygen in your own kitchen...
Warnings...
Oxygen is very very flammable.
Hydrogen can be explosive. (one word... Hindenburg -thought that's not really conclusive either!!)
together Hydrogen and Oxygen together are used as rocket fuel, -you've been warned... -popping the small steady stream of bubbles coming off the top seems perfectly safe...
Collecting the gasses in a large space full of the gasses and then setting light to them will probably not be a great thing to do.
(probably more to do with the oxygen than the hydrogen).
Other warnings.
You can put more power than 6 volts into the contraption. (and it does bubble faster).
I put 24 volts in using the -12 and 12 volt power rails from a PC power supply,
that power supply is now dead, I didn't bother to measure the resistivity to find out what sort of current would be drawn... now I need to make a (another) bench supply...
What you will have noticed is.
Hydrogen burns, Oxygen burns, but from tap water, with a simple 5, 6 or 12volts source it's not generated in significant quantities.
and that's exactly why water electrolysis machines that bolt onto cars just don't work. you don't get nearly enough gas to actually run an engine.
splitting the liquid into it's component forms takes more energy that you get from burning the gasses! so it's never going to power your car, or save you fuel.
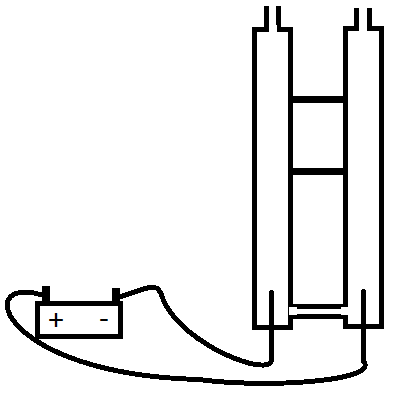
A little more complicated set-up can separate the gasses, what you need is two tubes, that are connectted at the bottom (below the electrodes) so that electricity can flow through the water, but the rising gasses won't go through the connection.
At this point Hydrogen will bubble up one tube (ready for you to collect in the top), Oxygen will bubble up the other tube. taps on the top of the tubes can seal them such that the gasses can't escape until you're ready to release the gasses.
A long tube connected to the bridge at the bottom can be used to top up the water level as you generate the gasses.
doing this in either glass or plastic will require special attention to make it all leak proof and gass tight of course.
Here's a rough picture of what your set-up will end up like.